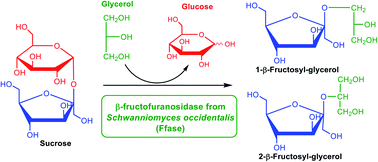
The β-fructofuranosidase from the yeast Schwanniomyces occidentalis (Ffase) produces potential prebiotic fructooligosaccharides (FOS) by self-transfructosylation of sucrose, being one of the highest known producers of 6-kestose. The use of Green Solvents (GS) in biocatalysis has emerged as a sustainable alternative to conventional organic media for improving product yields and generating new molecules. In this work, the Ffase hydrolytic and transfructosylating activity was analysed using different GS, including biosolvents and ionic liquids. Among them, 11 were compatible for the net synthesis of FOS. Besides, two glycerol derivatives improved the yield of total FOS. Interestingly, polyols ethylene glycol and glycerol were found to be efficient alternative fructosyl-acceptors, both substantially decreasing the sucrose fructosylation. The main transfer product of the reaction with glycerol was a 62 g L−1isomeric mixture of 1-O and 2-O-β-d-fructofuranosylglycerol, representing 95% of all chemicals generated by transfructosylation. Unexpectedly, the non-terminal 2-Ofructo-conjugate was the major molecule catalysed during the process, while the 1-Oisomer was the minor one. This fact made Ffase the first known enzyme from yeast showing this catalytic ability. Thus, novel fructosylated compounds with potential applications in food, cosmetics, and pharmaceutical fields have been obtained in this work, increasing the biotechnological interest of Ffase with innocuous GS.
Ref.:"Enzymatic synthesis of novel fructosylated compounds by Ffase from Schwanniomyces occidentalisin green solvents".David Piedrabuena, Ángel Rumbero, Elísabet Pires, Alejandro Leal-Duaso ,Concepción Civera, María Fernández-Lobato and Maria J. Hernaiz . RSC Advances, 11, 39, 24312-24319 (2021), doi: 10.1039/d1ra01391b