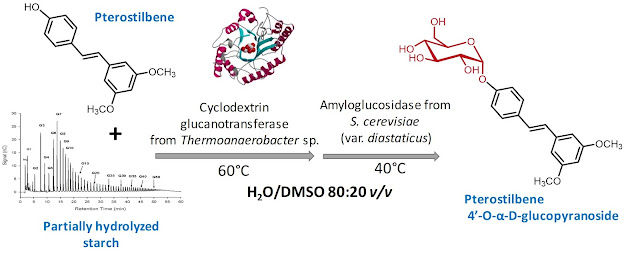
The synthesis of a novel α-glucosylated derivative of pterostilbene was performed by a transglycosylation reaction using starch as glucosyl donor, catalyzed by cyclodextrin glucanotransferase (CGTase) from Thermoanaerobacter sp. The reaction was carried out in a buffer containing 20% (v/v) DMSO to enhance the solubility of pterostilbene. Due to the formation of several polyglucosylated products with CGTase, the yield of monoglucoside was increased by the treatment with a recombinant amyloglucosidase (STA1) from Saccharomyces cerevisiae (var. diastaticus). The monoglucoside was isolated and characterized by combining ESI-MS and 2D-NMR methods. Pterostilbene α-d-glucopyranoside is a novel compound. Pterostilbene α-d-glucopyranoside was less toxic than pterostilbene for human SH-S5Y5 neurons, MRC5 fibroblasts and HT-29 colon cancer cells, and similar for RAW 264.7 macrophages.
Ref: J.L. González-Alfonso, D. Rodrigo-Frutos, E. Belmonte-Reche, P. Peñalver, A. Poveda, J. Jimenez-Barbero, A.O. Ballesteros, Y. Hirose, J. Polaina, J.C. Morales, M. Fernández-Lobato and F. J. Plou. Enzymatic synthesis of a novel pterostilbene α-glucoside by the combination of cyclodextrin glucanotransferase and amyloglucosidase. Molecules 23(6), 1271 (2018). https://doi.org/10.3390/molecules23061271